electron configuration sr|How to write the electron configuration for Strontium (Sr). : Tagatay Symbol: Sr; Atomic weight: 87.62; Electron shell configuration: [Kr]5s^2; Melting Point (K): 1042; Boiling Point(K): 1657
The city’s iconic tower houses, adorned with intricate white patterns, rise majestically against the azure sky. This makes it one of the best places to visit in Yemen. The Old City serves as a living example of why Sana’a is frequently listed as one of Yemen’s most picturesque cities.
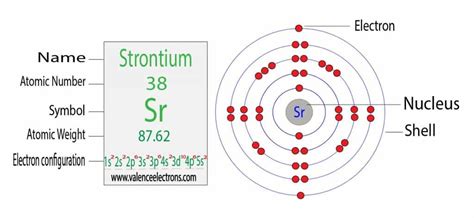
electron configuration sr,Strontium is a highly reactive element. When Strontium is exposed to air it makes a dark oxide layer. Electron configuration for Sr . In order to write the Sr electron configuration we first need to know the number of electrons for the Sr atom (there are 38 electrons). When we write the .Strontium is an alkaline earth metal with symbol Sr and atomic number 38. Its electron configuration is [Kr] 5s 2, which means it has two valence electrons in the outermost shell.
Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row . How to write the electron configuration for Strontium (Sr). Watch on. Methods. Aufbau principle. First, find electrons of strontium atom. Periodic table | Image: Learnool. The atomic number of strontium . Symbol: Sr; Atomic weight: 87.62; Electron shell configuration: [Kr]5s^2; Melting Point (K): 1042; Boiling Point(K): 1657How to write the electron configuration for Strontium (Sr). The chemical symbol for Strontium is Sr. Electron Configuration and Oxidation States of Strontium. Electron configuration of Strontium is [Kr] 5s2. Possible . To write the configuration for the Strontium ions, first we need to write the electron configuration for just Strontium (Sr). We first need to find the numb.The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to . An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully .electron configuration sr How to write the electron configuration for Strontium (Sr).Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 86 Sr, 87 Sr, 88 Sr Electron configuration [Kr] 5s 2 CAS number: 7440-24-6 ChemSpider ID: 4514263:

To write the configuration for the Strontium ions, first we need to write the electron configuration for just Strontium (Sr). We first need to find the numb.

To write the configuration for the Strontium ions, first we need to write the electron configuration for just Strontium (Sr). We first need to find the numb.electron configuration sr The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number .Strontium is a chemical element of the periodic table with chemical symbol Sr and atomic number 38 with an atomic weight of 87.621 u and is classed as a alkaline earth metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 5s 2: Electrons per shell: 2, 8, 18, 8, 2: Valence electrons : 2: Valency electrons : 2:The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .Strontium. Full electron configuration of strontium: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2 rubidium ← strontium → yttrium. © 2009-2018 | www.prvky.com . Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from .
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .
Electron shell configuration: [Kr]5s^2; Melting Point (K): 1042; Boiling Point(K): 1657; Introduction. The element strontium is named for a Scottish town, Strontian. It was isolated in 1808 by Davy and is a silvery and malleable metal that reacts vigorously with water to produce hydrogen gas. . The isotope 90 Sr is one of the world's best .Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. . Strontium (Sr) 38: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2: Yttrium (Y) 39: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 1: Zirconium (Zr) 40: 1s 2 2s 2 2p 6 3s . The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save . The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic .Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .Hydrogen and helium are placed somewhat arbitrarily. Although hydrogen is not an alkali metal, its 1s 1 electron configuration suggests a similarity to lithium ([He]2s 1) and the other elements in the first column.Although helium, with a filled ns subshell, should be similar chemically to other elements with an ns 2 electron configuration, the closed .
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 .
electron configuration sr|How to write the electron configuration for Strontium (Sr).
PH0 · Strontium electron configuration
PH1 · Strontium Electron Configuration (Sr) with Orbital Diagram
PH2 · Strontium Electron Configuration (Sr) with Orbital
PH3 · Strontium (Sr)
PH4 · Strontium
PH5 · How to write the electron configuration for Strontium (Sr).
PH6 · How to Write the Electron Configuration for the Sr 2
PH7 · Complete Electron Configuration for Strontium (Sr, Sr2+)
PH8 · Chemistry of Strontium (Z=38)
PH9 · 2.4 Electron Configurations